
Enable R&D Teams
to Innovate Faster
Improve team efficiency and collaboration by simplifying workflows and centralizing assets for faster project execution.
5000+ Brands















Bridge innovation and execution with precise artwork management
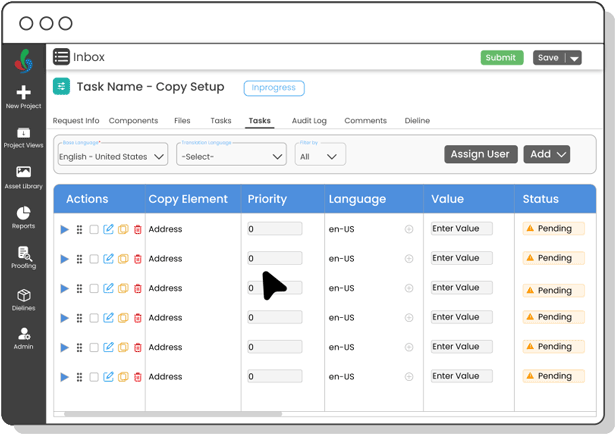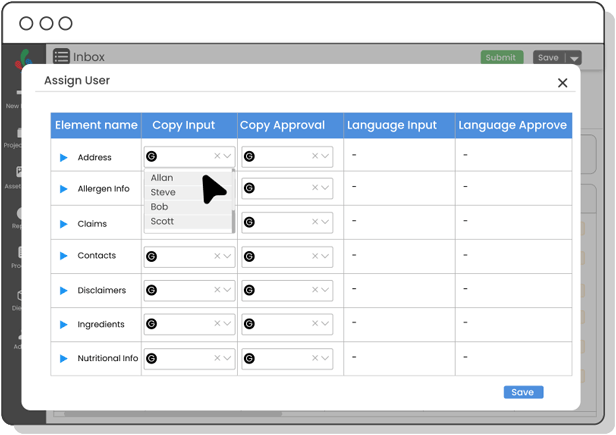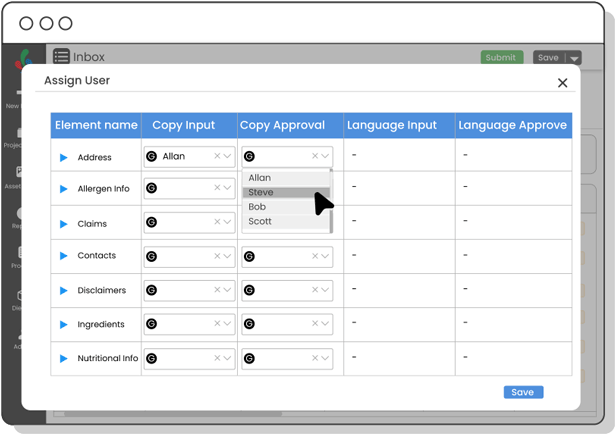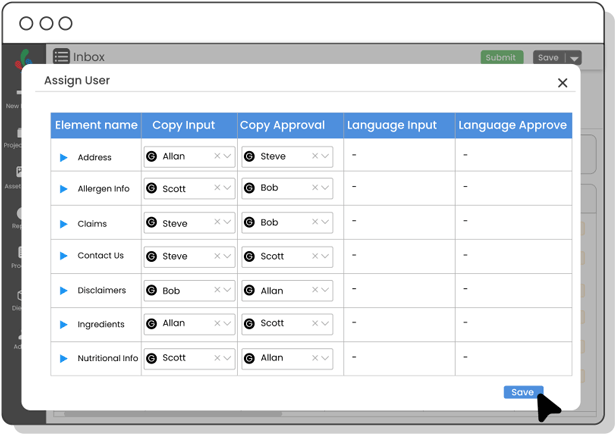
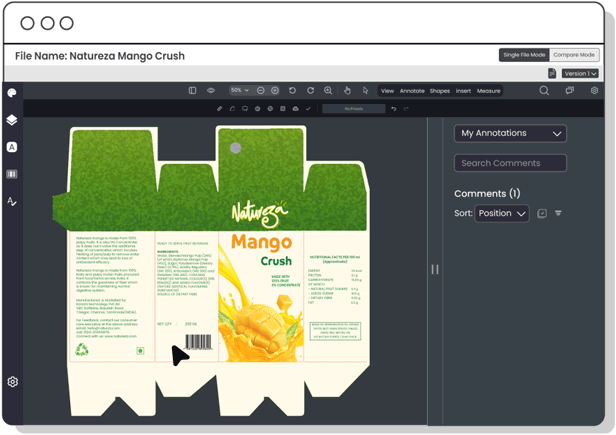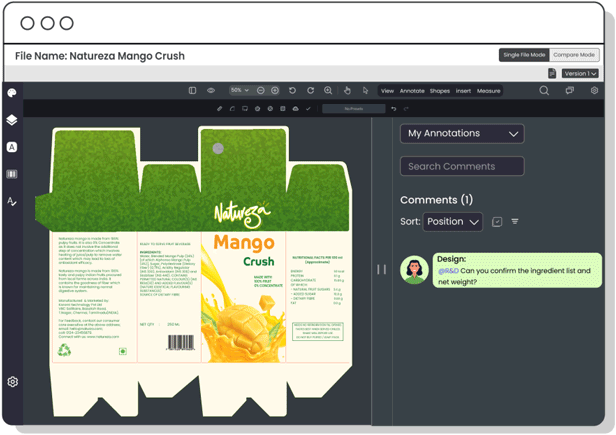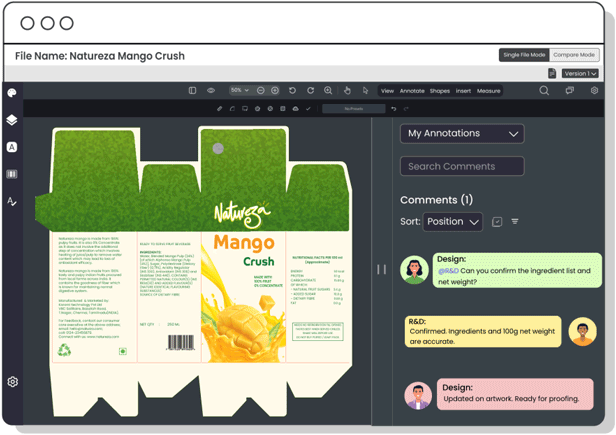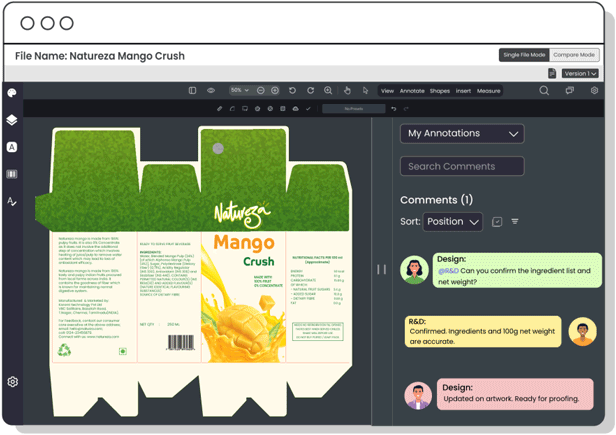
Copy Manager
Centralize packaging copy and ensure each content element is approved—minimizing errors, improving update speed, and maintaining control over claims, warnings, and ingredient details.
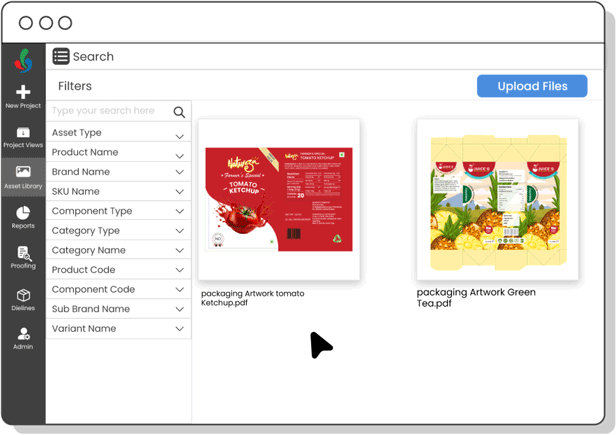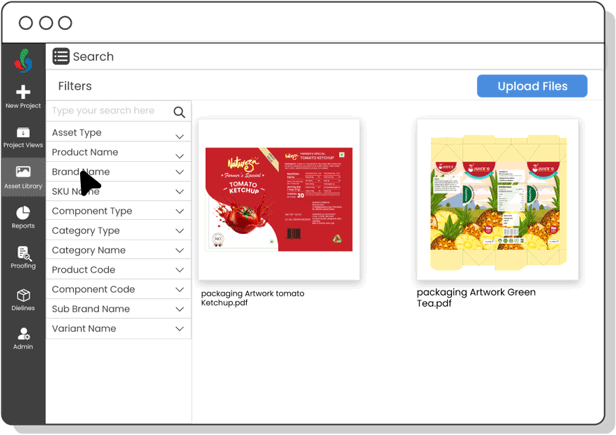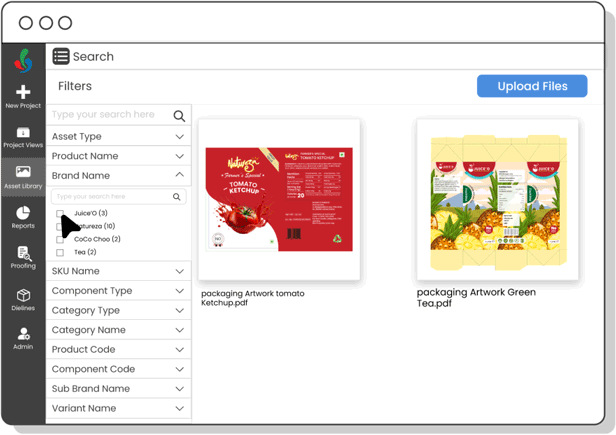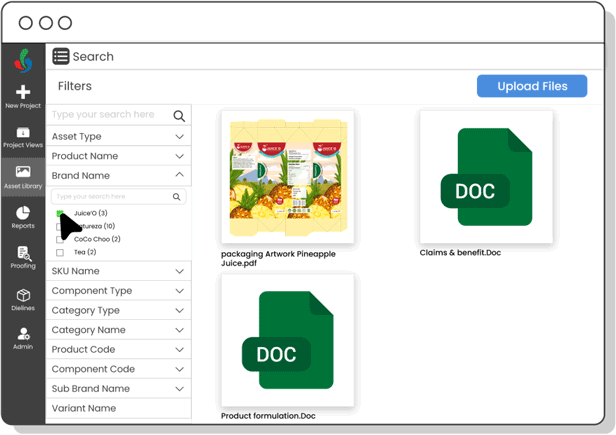
Digital Asset Management
Organize and track artwork assets efficiently—ensuring fast access to current files, preventing outdated usage, and speeding up R&D development cycles.
Streamlined Workflows
Accelerate R&D collaboration with efficient, integrated workflows—removing manual steps, reducing delays, and helping teams focus on innovation and meet deadlines.
Drive innovation with smarter packaging artwork processes
Streamline Collaboration on Formula and Packaging Integration
Centralize communication between R&D, packaging, and design teams to seamlessly integrate product formulations with packaging needs, ensuring accurate product representation, avoiding last-minute changes, and maintaining regulatory compliance.


Facilitate Faster Product Development Cycles
Accelerate product innovation by streamlining packaging iterations. With quick access to templates, artwork versions, and centralized data, R&D teams can reduce packaging approval cycle time, supporting rapid prototyping without compromising quality or compliance.
Ensure Data Accuracy and Consistency Across Teams
Consolidate technical data, such as nutritional values and product specifications, in a centralized platform. This allows R&D teams to share verified details with other teams, ensuring accurate, error-free, regulation-compliant packaging.


Adapt Packaging to Evolving Market Needs
Respond swiftly to market trends or regulatory updates with agile packaging workflows. R&D teams can update formulations, ingredient disclosures, and specifications, seamlessly integrating these changes into packaging designs to align with evolving market demands.
Centralize Data for New Product Initiatives
Centralize technical data, testing results, and packaging materials to simplify access to historical information. This reduces duplication of effort, streamlines workflows, and enables R&D teams to launch innovative packaging solutions more quickly.

Things You Might Be Wondering About
Can I manage all my packaging copy in one place?
Yes! With Copy Manager, you can centralize every bit of packaging text—ingredients, claims, warnings—and track approvals at the element level.
Does the system help prevent copy errors before launching?
Absolutely. Each content block goes through a structured approval flow, so what ends up on your packaging is exactly what was approved—no surprises.
Can I access older artwork versions during development?
Yes, every version is saved. You can pull up past iterations, spot differences, or restore earlier files whenever needed.
Can I avoid delays when formula or packaging specs change?
Updates are made in one place and instantly reflected across teams—so packaging, design, and regulatory are always on the same page.
Can I speed up approvals for new product development?
Definitely. Our workflow tools streamline reviews and alerts, helping you cut down packaging approval cycles without missing details.
Can the system support evolving market or regulatory needs?
Yes. When ingredient lists, claims, or disclosures change, the system helps you update and approve them quickly, keeping your packaging market ready.
Still have questions?
Can’t find the answer you’re looking for? Please chat to our friendly team.